January 22, 2020 feature
Multivariate patterning of human pluripotent cells reveals induced paracrine factors in kidney organoid development

Thamarasee Jeewandara
contributing writer
Bioengineers have shown great promise in creating complex multicellular kidney organoids (tiny, self-organized tissues) in the lab using pluripotent stem cells . They can further improve the procedure for different outcomes, including patterning and maturation of specific cell types, although such experiments are limited by standard tissue culture approaches. Now writing in Science Advances, Nick R. Glass and an interdisciplinary research team at the Australian Institute for Bioengineering and Nanotechnology, Murdoch Children's Research Institute and the Department of Pediatrics and Biomedical Manufacturing in Australia detailed a new full factorial microbioreactor array-based method to perform complex in the lab.
Using the method, they rapidly interrogated and optimized the complex cell differentiation and development process in a simplistic setup. Glass et al. successfully recapitulated early kidney tissue patterning events and explored more than 1000 unique conditions to achieve near-pure renal cell type specification. The team conducted single-cell resolution identification of distinct renal cell types within multilayered kidney organoids and coupled the results with to define the roles of several signaling proteins. The bioengineers also noted (RA) as a minimal effect of nephron patterning, while highlighting contributions of induced on cell specification and patterning.
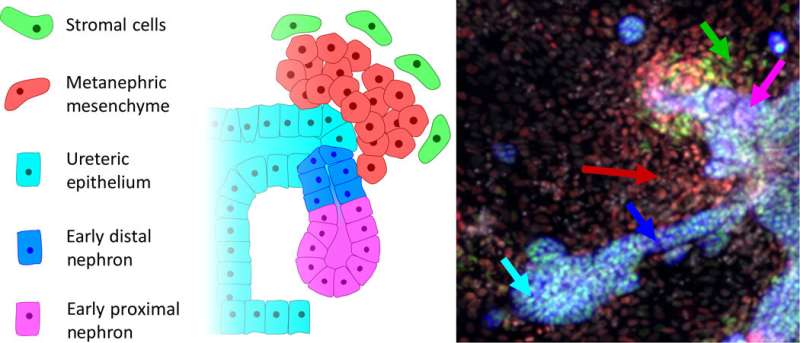
The mammalian kidney is formed during early embryonic development from two different types; one to generate and the other to form functional filtering units of the kidney known as . More specifically, the mammalian kidney is a byproduct of the (IM), arising through between several key (stem cell-like) IM subpopulations. While the anterior IM-derived epithelial nephric duct formed the and collecting ducts of the kidney. The filtering units of the kidney or nephrons typically arose via epithelial transformations of the posterior IM known as the (MM). Scientists have studied normal mammalian kidney development to for directed differentiation of (hPSCs) and engineer to understand kidney disease. While most protocols formed primitive patterns of the trunk mesoderm, further procedures resulted in early nephron generation in the lab.
For instance, after seven days of monolayer culture under three-dimensional (3-D) culture conditions, cells could self-assemble to form containing segmented nephrons, epithelium, endothelial progenitors and a surrounding . Static culture conditions did not, however, allow investigators to probe direct, synergistic or influences governing cell fate specification, therefore they used expensive infrastructure such as conditions in the lab.
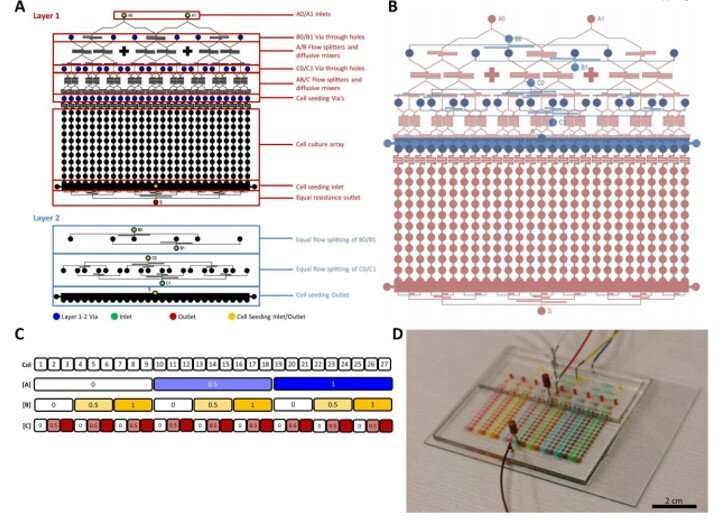
Even still, the intrinsic static nature of the devices remained limiting, so to overcome limits, they used microfabricated devices with successful applications across ; facilitating unprecedented investigations into complex biological phenomena. Research teams had also previously designed microdevices to and simultaneously conduct many experiments . These microbioreactor arrays (MBAs) offered them opportunity to increase parameter space and reduce timeframes of .
In this work, Glass et al. detailed applications of the MBA-based method to achieve unbiased, factorial assessment of stem cell-derived IM progenitor differentiation in to multiple renal cell lineages, followed by patterning them into multicellular kidney organoids. They developed a custom post-processing algorithm to quantitatively analyze the outputs of image cytometry to their phenotype—at single cell resolution. The new MBA platform validated previous protocols on renal lineage regeneration and identified previously unappreciated critical impacts of paracrine signalling during the process. Glass et al. specified roles for (BMP7) and prolonged (a group of signal transduction pathways) during renal cell fate decisions and organoid patterning.
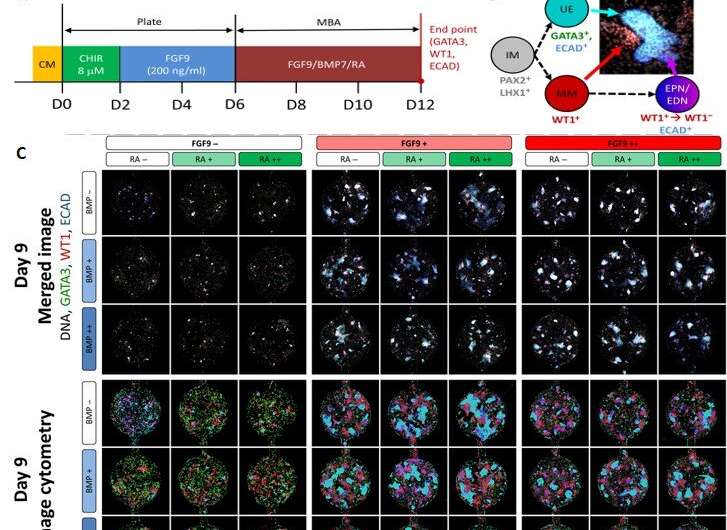
During the experiments, the team seeded the MBA with six-day old IM progenitors to produce human embryonic stem cells (HES3 cells) and treated the culture with a Wnt agonist . After cell culture for 16 hours, Glass et al. investigated the IM progenitors via gene expression studies and observed cell aggregates that formed a colony-like structure within the wells in time. The team then tested the influence of three molecules; (FGF9), bone morphogenetic protein 7 (BMP7) and retinoic acid (RA) due to their in kidney cell type induction. However, the combined interplay of these factors remained unknown.
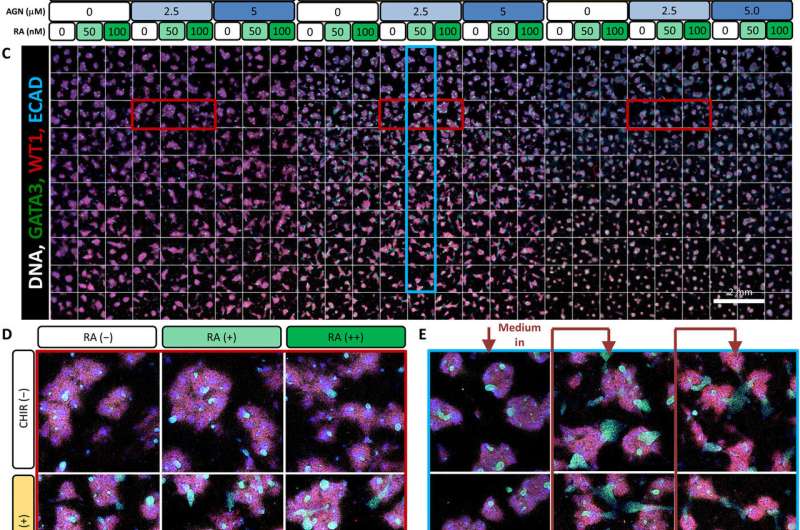
Using the nested resistive network within the MBA, Glass et al. split and recombined input media of the three factors (FGF9, BMP7 and RA) at different concentrations for a total of nine or 12 days. At both timepoints, they fixed the cells, counted them and identified them (phenotyped them) using to readily identify distinct cell types belonging to the early mammalian embryonic kidney. The 12-day culture led to significantly more complex and mature structures. The team conducted analysis to understand the influence of the three factors; showing FGF9 as a vital factor for cell proliferation and maintenance of all cell types.
Similarly, BMP7 increased the total cell number while affecting the proportion of cell specification during early kidney differentiation. Based on the levels of BMP7 in the presence of FGF9, and in response to paracrine signaling, Glass et al. recorded substantial variations in the (structure) within the wells. However, RA did not appear to play a role during early kidney differentiation, which apparently depended on the short, two-day exposure to at the onset of cell culture.
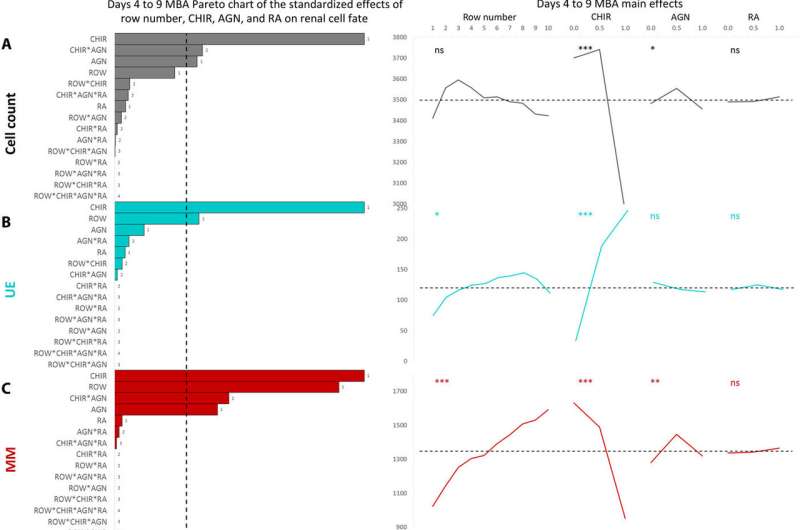
In this way, Nick R. Glass and colleagues showed the capacity to precisely direct stem cell differentiation in the lab to form kidney tissue and individual kidney cell types as a major step towards applications in and drug discovery. Since paracrine-mediated differentiation events which direct renal cell type specification can be difficult to replicate in standard static culture conditions, Glass et al. used an MBA (microbioreactor array) platform to investigate renal cell fate. The data showed that small-step changes in exogenous FGF9, BMP7 and CHIR, as well as endogenous signaling in the form of paracrine and autocrine factors, could shift the ratio of cell differentiation.
As a result, they formed ureteric, metanephric mesenchyme (MM), early proximal nephrons (EPNs) and distal nephron (DN) phenotypical cells, starting from IM precursors. Additionally, FGF9 signaling was critical to maintain all cell populations. The step-wise patterning that occurred in the MBA, was similar to the processes in the embryo, where multiple, distinct cell types that signal to each other arose across time. The MBA-based factorial approach can be applied to obtain complex, multistep differentiation for multicellular outcomes and the process will be able to tune differentiation of other organoid-like pluripotent stem-cell derived tissues.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Nick R. Glass et al. Multivariate patterning of human pluripotent cells under perfusion reveals critical roles of induced paracrine factors in kidney organoid development, Science Advances (2020).
M. Takasato et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney, Nature Cell Biology (2013).
Minoru Takasato et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis, Nature (2015).
Journal information: Science Advances , Nature Cell Biology , Nature
© 2020 Science X Network













