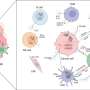
Schematic illustration of the preparation route and anti-tumor mechanism of pLCGM-OVA. Credit: LI Qingdong
Researchers have developed an innovative nano-immune agonist that significantly improves immunotherapy outcomes for melanoma—a highly aggressive and hard-to-treat form of skin cancer.
The , published in the Journal of Colloid and Interface Science, introduces a biodegradable therapeutic nanomaterial called pLCGM-OVA. The team included Prof. Wu Zhengyan from the Hefei Institutes of Â鶹ÒùÔºical Science of the Chinese Academy of Sciences, in collaboration with Prof. Zhang Guilong from Binzhou Medical University.
Melanoma remains a clinical challenge largely due to its strong immunosuppressive tumor microenvironment, which weakens the effectiveness of conventional immune checkpoint inhibitors. To overcome this hurdle, the researchers designed a multifunctional nanoplatform capable of reshaping the tumor microenvironment and enhancing the body's natural immune defense against cancer.
The team designed and synthesized a safe and efficient nano-immune agonist. It works through a combination of mechanisms: it induces immunogenic cell death via reactive oxygen species and copper-triggered cuproptosis—a newly recognized form of regulated cell death.
Additionally, it incorporates ovalbumin (OVA) as a model antigen to boost vaccine-like immune responses, and importantly, it activates the cGAS-STING signaling pathway, a key player in innate immune activation. Together, these effects stimulate a strong anti-tumor immune response, effectively suppressing both melanoma growth and recurrence.
Beyond its therapeutic capabilities, pLCGM-OVA also serves as a T1-weighted magnetic resonance imaging (MRI) contrast agent, enabling simultaneous diagnosis and treatment—a promising step toward advanced cancer theranostics.
This research marks a significant advancement in nanomedicine and cancer immunotherapy, offering new hope for more effective melanoma treatments.
More information: Qingdong Li et al, Biodegradable nano-immune agonist for enhanced immunotherapy of melanoma via the synergistic action of cuproptosis and cGAS-STING enhanced immune response, Journal of Colloid and Interface Science (2025).
Provided by Chinese Academy of Sciences