Cryo-em freezes the funk: Scientists visualize a pungent protein

Lisa Lock
scientific editor

Robert Egan
associate editor

They say a picture is worth a thousand words. But it takes millions of pictures to understand the intricate chemistry of an enzyme that helps break down sulfur, commonly found in fruits, vegetables, alcohol and gasoline, into the colorless gas most noted for its distinctive odor.
Most people have witnessed—or rather smelled—when a protein enzyme called sulfite reductase works its magic. This enzyme catalyzes the chemical reduction of sulfite to hydrogen sulfide. Hydrogen sulfide is the rotten egg smell that can occur when organic matter decays and is frequently associated with sewage treatment facilities and landfills.
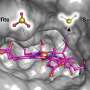
But scientists have not been able to capture a visual image of the enzyme's structure until now, thus limiting their full understanding of how it works. Florida State University Professor of Biological Science Elizabeth Stroupe and her former doctoral student Behrouz Ghazi Esfahani have solved that problem and their work in the journal Nature Communications.
"Artificial intelligence has gotten better at predicting protein structures, but at the end of the day, it's not data," Stroupe said. "This gives us the primary knowledge we need to better understand this kind of structure."
Stroupe and Ghazi Esfahani used an advanced technique called cryo-electron microscopy to visualize the 3D structure of this enzyme. Cryo-electron microscopy allows scientists to continually capture images of chemical reactions, giving them the necessary data to visualize the structure.
To the untrained eye, protein molecules look like complicated strings of chemicals, but this clear visualization of the 3D structure allows scientists to see the exact arrangement of atoms and how electron transfer occurs.
"I think of it as an octopus with four yo-yos because the molecule is particularly flexible," Stroupe said.
This work is essential for scientists so they can learn how to control or manipulate chemical reactions, a process that is often used by drug manufacturers or industry when they develop products with these chemicals.
"There are environmental implications too," Ghazi Esfahani said. "Some bacteria use sulfur as an energy source the way humans or other living creatures use oxygen. This allows us to understand how some of those bacteria thrive in anaerobic conditions."
This research was a big step in gaining a better understanding of how sulfite reductase works, but there are still unanswered questions about how it functions as a larger protein assembly and how similar enzymes in other organisms, like the pathogen that causes tuberculosis, which depends on sulfur to live in a human host, work.
Stroupe's lab is continuing to work on that problem as well as other structural questions related to the sulfur metabolism process.
More information: Behrouz Ghazi Esfahani et al, Structure of dimerized assimilatory NADPH-dependent sulfite reductase reveals the minimal interface for diflavin reductase binding, Nature Communications (2025).
Journal information: Nature Communications
Provided by Florida State University