How morphogens steer early brain development by guiding stem cell gene activity

Just a few weeks after conception, stem cells are already orchestrating the future structure of the human brain. A new Yale-led study shows that, early in development, molecular "traffic cops" known as morphogens regulate the activation of gene programs that initiate stem cells' differentiation into more specialized brain cells.
The Yale team found that sensitivity to these signaling morphogens can vary not only between stem cells from different donors, but between stem cells derived from the same individual.
"This is a new chapter in understanding how we develop and how development can be influenced by genomic changes between people and by epigenetic modifications within individuals," said Flora Vaccarino, the Harris Professor in the Child Study Center at the Yale School of Medicine (YSM) and co-senior author of the research, in the journal Cell Stem Cell.
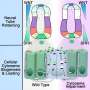
The team, led by Vaccarino and co-senior author Andre Levchenko, the John C. Malone Professor of Biomedical Engineering at the Yale School of Engineering & Applied Science and at YSM, developed a device called Duo-MAPs, which allowed them to expose organoids derived from human stem cells to two crucial morphogens naturally present within the developing brain.
The WNT morphogen, active along the posterior-anterior (bottom to top) axis of the nascent central nervous system, interacts with the Sonic Hedgehog morphogen, which operates along ventro-dorsal (front to back) axis of developing nervous system.
Together, the location and concentrations of the two morphogens over just 5 days regulated the gene activity that determined the eventual structure and cell composition of almost all brain regions, the researchers found.
Intriguingly, the high-throughput analysis enabled by the device showed distinct differences in the two morphogens' gene activity in organoids derived from different individuals and different stem cell lines. For instance, organoids from some stem cell lines showed higher sensitivity to the WNT morphogen and the activated genes were concentrated towards the bottom of the brain where the hindbrain develops.
Other lines showed lower sensitivity to WNT and activity shifted toward anterior or frontal brain areas, such as the developing cortex. Similarly, stem cell lines more sensitive to Sonic Hedgehog showed higher gene activity in the developing basal ganglia, while stem cells less sensitive to the morphogen had a greater gene response in the developing cerebellum. The morphogen-response genes that were most variable among different donors involved functions such as immune response, other experiments showed.
Surprisingly, morphogen-elicited gene activity varied among different cell lines derived from a single individual. Other genes involved in cell metabolism fluctuated from one experimental prep to another in the same cell lines, the authors said.
The variable response patterns across donors are likely driven by their genetic background, the authors found. However, variations in the responses to morphogens in stem cell lines from the same donor are likely caused by epigenetic changes or post-conception mutations carried by each line.
Altogether, the findings indicate the fluid nature of brain development across people and even within the same individual.
"It was striking to see that the human brain development can be triggered by a relatively short exposure to two key signals and that it is apparently very robust to variation of gene expression," Levchenko, who is also director of the Systems Biology Institute at Yale's West Campus, said.
"This research opens the door to a more compressive modeling and understanding of a key developmental process that can be linked to specific human subjects in a much more precise manner than before."
More information: Soraya Scuderi et al. Specification of human brain regions with orthogonal gradients of WNT and SHH in organoids reveals patterning variations across cell lines, Cell Stem Cell (2025). .
Journal information: Cell Stem Cell
Provided by Yale University