Mist and sea spray create unique conditions for urea to form from simple gases

Stephanie Baum
scientific editor

Andrew Zinin
lead editor

Urea is considered a possible key molecule in the origin of life. ETH researchers have discovered a previously unknown way in which this building block can form spontaneously on aqueous surfaces without the need for any additional energy.
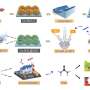
Urea is one of the most important industrial chemicals produced worldwide. It is used as a fertilizer, for the production of synthetic resins and explosives and as a fuel additive for cleaning car exhaust gases. Urea is also believed to be a potential key building block for the formation of biological molecules such as RNA and DNA in connection with the question of the origin of life.
Until now, the origin of urea itself on early Earth has not been conclusively clarified.
A research team led by Ruth Signorell, Professor of Â鶹ÒùÔºical Chemistry at ETH Zurich, has discovered a previously unknown reaction pathway for the formation of urea that could provide an answer. The study in the journal Science.
Chemistry on the water surface
Either high pressures and temperatures or chemical catalysts are needed for the industrial production of urea from ammonia (NH₃) and carbon dioxide (CO₂). Enzymes enable the same reaction to take place in humans and animals, removing toxic ammonia from the breakdown of proteins. As this simple molecule contains nitrogen as well as carbon and probably existed on the uninhabited early Earth, many researchers view urea as a possible precursor for complex biomolecules.
"In our study, we show one way in which urea could have formed on the prebiotic Earth," says Signorell, "namely where water molecules interact with atmospheric gases: on the water surface."
Reactor on the edge of a droplet
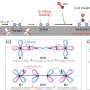
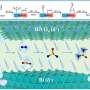
Signorell's team studied tiny water droplets such as those found in sea spray and fine mist. The researchers observed that urea can form spontaneously from carbon dioxide (CO₂) and ammonia (NH₃) in the surface layer of the droplets under ambient conditions. The physical interface between air and liquid creates a special chemical environment at the water surface that makes the spontaneous reaction possible.
As a droplet has a very large surface area in relation to its volume, chemical reactions mainly take place near this surface. Chemical concentration gradients form in this area, which acts like a microscopic reactor. The pH gradient across the interfacial layer of the water droplets creates the required acidic environment, which opens unconventional pathways that would otherwise not take place in liquids.
"The remarkable aspect of this reaction is that it takes place under ambient conditions without any external energy," explains Mercede Mohajer Azizbaig, one of the two first authors. This not only makes the process interesting from a technical perspective but also provides valuable insights into processes that could be significant for evolution.
A window into the early days of the Earth
The origin of life is currently the subject of a great deal of wide-ranging research, with different approaches being explored.
First author Pallab Basuri explains, "Given such a controversial field of research, it was important for us to back up our observations."
Theoretical calculations by co-authors Evangelos Miliordos and Andrei Evdokimov from Auburn University supported the experimental findings and confirmed that the urea reaction on the droplets takes place without any external energy supply.
The results suggest that this natural reaction could also have been possible in the atmosphere of early Earth—an atmosphere that was rich in CO₂ and probably contained small traces of ammonia. In such environments, aqueous aerosols or fog droplets could have acted as natural reactors in which precursor molecules such as urea were formed.
"Our study shows how seemingly mundane interfaces can become dynamic reaction spaces, suggesting that biological molecules may have a more common origin than was previously thought," says Signorell.
In the long term, the direct reaction of COâ‚‚ and ammonia under ambient conditions could also have potential for climate-friendly production of urea and downstream products.
More information: Mercede Azizbaig Mohajer et al, Spontaneous formation of urea from carbon dioxide and ammonia in aqueous droplets, Science (2025).
Journal information: Science
Provided by ETH Zurich