Study clarifies catalyst design for cleaner ammonia production

Sadie Harley
scientific editor

Andrew Zinin
lead editor

Researchers at Tohoku University have uncovered key principles that could advance sustainable ammonia production by electrochemically converting nitrate waste.
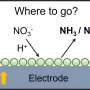
Their findings clarify how the coordination structure of metal-nitrogen-carbon (M-N-C) catalysts influences their performance in the nitrate reduction reaction (NO3RR), a process that can potentially treat agricultural runoff while producing ammonia for fertilizers.
Ammonia production today relies heavily on energy-intensive industrial processes. By contrast, the electrochemical approach offers a path to generate ammonia under ambient conditions using renewable electricity.
"We systematically compared pyrrolic- and pyridinic-coordinated M-N-C catalysts and found that pyrrolic structures generally achieve higher turnover frequencies for ammonia production," explained Hao Li, who led the study.
"Our analysis also revealed that the adsorption and protonation of nitrate—often overlooked in previous models—are in fact the rate-determining steps in this reaction."
The team combined systematic data analysis of over 60 catalysts with pH-field coupled microkinetic modeling on a reversible hydrogen electrode scale. Their subsequent experiments under both neutral and alkaline conditions validated the theoretical predictions.

"The results challenge the classical thermodynamic 'limiting-potential model' used in many prior studies, showing it does not accurately capture the catalytic performance trends for different M-N-C materials," said Li. "This opens up new design strategies for more efficient catalysts."
Looking ahead, the group plans to develop machine learning potentials capable of modeling electric field interactions with weakly-adsorbed species such as *NO3H and *NO2H. This will support automated screening of diverse M-N-C configurations to identify promising candidates for nitrate-to-ammonia conversion.

All data from this study are available through the , the largest experimental catalysis database developed by Hao Li Lab.
"This work is part of our broader goal to create better tools for catalyst design," noted Li. "Ultimately, we hope to contribute to cleaner, more sustainable ammonia production technologies that benefit both industry and the environment."
More information: Qiuling Jiang et al, The Key Steps and Distinct Performance Trends of Pyrrolic vs Pyridinic M–N–C Catalysts in Electrocatalytic Nitrate Reduction, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by Tohoku University