Common metal, unusual power: Manganese complex sets new standard in photochemistry

Gaby Clark
scientific editor

Robert Egan
associate editor

Reactions are typically driven by heat. However, in recent years, light has also established itself as an energy source, as it allows chemical reactions to be controlled with exceptional precision. This process is known as photochemistry.
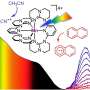
The problem: Until now, this kind of reaction required ruthenium, osmium, or iridium—materials that are both rare and expensive, as well as environmentally harmful when mined. A research team at Johannes Gutenberg University Mainz (JGU) has developed a novel metal complex based on the abundant and inexpensive manganese.
"This metal complex sets a new standard in photochemistry: it combines a record-breaking excited-state lifetime with simple synthesis," stated Professor Katja Heinze from the JGU Department of Chemistry. "It thus offers a powerful and sustainable alternative to the noble metal complexes that have long dominated light-driven chemistry."
The results have been recently in Nature Communications.
Single-step synthesis and strong absorption
Manganese is over 100,000 times more abundant on Earth than the noble metal ruthenium, but its application in photochemistry has been severely limited to date: firstly, by the tedious, multi-step synthesis, which often requires nine to ten steps, and, secondly, by the short lifetime of the excited state.
"The newly developed manganese complex overcomes both challenges," explained Dr. Nathan East, a former doctoral student in the Heinze group who carried out the original synthesis. The new material is synthesized directly from commercially available starting materials—in just a single synthesis step.
In addition to manganese, the researchers use a ligand, which allows the properties of the complex to be tuned. "The combination of a colorless manganese salt and the colorless ligand in solution immediately produces a deep purple color, just like ink. This is a very unusual color for a manganese complex, which showed us that something unique was happening," added Sandra Kronenberger, who further investigated this novel manganese complex as a doctoral student in the Heinze group at the Max Planck Graduate Center (MPGC).
The resulting manganese complex not only looks impressive, it also exhibits remarkable properties. "Its light absorption is exceptionally strong, meaning the probability of capturing a light particle is very high—the complex thus uses light very efficiently," explained Dr. Christoph Förster, who supported the project with quantum chemical calculations.
Excited state lifetime exceeds the 190-nanosecond mark
"The lifetime of the complex of 190 nanoseconds is also remarkable. This is two orders of magnitude longer than any previously known complexes containing common metals such as iron or manganese," said lead scientist and spectroscopist Dr. Robert Naumann, who characterized the dynamics of the excited state of the complex using luminescence spectroscopy.
In photochemistry, the catalyst, in this case the manganese complex, is excited by light. When it encounters another molecule through diffusion, it transfers an electron to it. Since it can take nanoseconds for the particles to find each other, the excited state must last as long as possible.
But does the complex actually do what the researchers hope it will, i.e., transfer an electron to another molecule? "We were able to detect the initial product of the photoreaction—the electron transfer that occurred—and thus prove that the complex reacts as desired," summarized Professor Heinze.
This discovery expands the boundaries of sustainable photochemistry. Thanks to its scalable one-step synthesis, efficient light absorption, robust photophysical behavior, and long-lasting excited state, the new manganese-containing material paves the way for future large-scale applications of photoreactions. This could be important for future applications like sustainable hydrogen production.
More information: Sandra Kronenberger et al, A manganese(I) complex with a 190 ns metal-to-ligand charge transfer lifetime, Nature Communications (2025).
Journal information: Nature Communications
Provided by Johannes Gutenberg University Mainz