Watching how accessory proteins regulate filament growth in real time

Lisa Lock
scientific editor

Robert Egan
associate editor

Using optical tweezers, researchers at National Taiwan University have observed individual binding events in real time, offering new insights into the molecular regulation of homologous recombination.
Their new published in Nucleic Acids Research reveals how accessory proteins regulate RAD51 filament growth, a critical step in homologous recombination and DNA repair.
The research, led by Prof. Hung-Wen Li (Department of Chemistry) and Prof. Peter Chi (Institute of Biochemical Sciences), bridges biophysics and biochemistry to address one of the central challenges in DNA repair: how RAD51 assembles on DNA in real time.
Traditional structural techniques capture only static or averaged views of protein clusters, leaving unanswered which oligomeric forms actively drive filament growth.
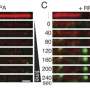
To overcome this, the team applied single-molecule optical tweezers, which use focused laser beams to manipulate DNA molecules attached to microscopic beads. As RAD51 proteins bound and extended along DNA, the resulting length changes were tracked with nanometer precision, enabling researchers to directly observe stepwise filament growth.
This approach uncovered a striking regulatory role of accessory proteins. RAD51 alone assembled primarily in octameric units, but in the presence of the SWI5-SFR1 complex, the assembly shifted to tetramers. This remodeling stabilized RAD51 filaments, making the assembly process more uniform and efficient.
These insights highlight how accessory proteins fine-tune recombination to safeguard genome integrity. Beyond advancing our understanding of DNA repair, the findings hold implications for cancer biology and genome editing technologies.
"This work demonstrates how single-molecule platforms, coupled with interdisciplinary collaboration, can illuminate fundamental biological processes in unprecedented detail," says Prof. Hung-Wen Li.
More information: Yingying Hu et al, SWI5–SFR1 reduces RAD51 recombinase extending units during filament assembly, Nucleic Acids Research (2025).
Journal information: Nucleic Acids Research
Provided by National Taiwan University