Engineered E. coli produce biodegradable plastic that outperforms widely used PET

Gaby Clark
scientific editor

Robert Egan
associate editor

The PET (polyethylene terephthalate)-alternative PDCA (pyridinedicarboxylic acid) is biodegradable and has superior physical properties, according to a recent study. A Kobe University team of bioengineers engineered E. coli bacteria to produce the compound from glucose at unprecedented levels and without byproducts—and opened up a realm of possibilities for the future of bioengineering. The findings are in the journal Metabolic Engineering.
The durability of plastics is both the reason why they have become so widespread and why they pose environmental problems. In addition, they are mainly sourced from petroleum, making them nonrenewable and contingent on geopolitics. Research groups worldwide work on both biodegradable and bio-sourced alternatives, but there often are issues with yield, purity and—as a result—associated production cost.
Kobe University bioengineer Tanaka Tsutomu says, "Most biomass-based production strategies focus on molecules consisting of carbon, oxygen and hydrogen. However, there are highly promising compounds for high-performance plastics that include other elements such as nitrogen, but there are no efficient bioproduction strategies. And purely chemical reactions inevitably generate unwanted byproducts."
PDCA is such a candidate. It is biodegradable, and materials incorporating this show physical properties comparable to or even surpassing those of PET, which is widely used in containers and textiles.
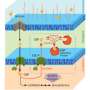
"Our group approached the challenge from a new angle: We aimed to harness cellular metabolism to assimilate nitrogen and build the compound from start to finish," says Tanaka.
The Kobe University group has now achieved the production of PDCA in bioreactors at concentrations more than seven-fold higher than previously reported.
Tanaka explains, "The significance of our work lies in demonstrating that metabolic reactions can be used to incorporate nitrogen without producing unwanted byproducts, thereby enabling the clean and efficient synthesis of the target compound."
The group, however, did have some stubborn problems to solve along the way. The most stubborn of these came when they discovered a bottleneck where one of the enzymes they had introduced produced the highly reactive compound hydrogen peroxide, H2O2. The compound then attacked the enzyme that produced it, thereby deactivating it.
"Through refining the culture conditions, in particular by adding a compound that can scavenge H2O2, we could finally overcome the issue, although this addition may present new economic and logistical challenges for large-scale production," says Tanaka.
The bioengineers already have plans on how to improve the production going forward, with every problem pointing the way to its solution. Looking at the future, Tanaka says, "The ability to obtain sufficient quantities in bioreactors lays the groundwork for the next steps toward practical implementation. More generally, though, our achievement in incorporating enzymes from nitrogen metabolism broadens the spectrum of molecules accessible through microbial synthesis, thus enhancing the potential of bio-manufacturing even further."
More information: Akinobu Katano et al, Biosynthesis of 2,5-pyridinedicarboxylate from glucose via p-aminobenzoic acid in Escherichia coli, Metabolic Engineering (2025).
Provided by Kobe University