Abstract. Credit: ACS Applied Materials & Interfaces (2025). DOI: 10.1021/acsami.5c11155
Fearless bacteria have colonized extreme environments, adapted to vast temperatures and pH fluctuations, and acclimated to diverse hosts. Among these multitudes of species is the exclusive club of good bacteria that have enormous benefits to humans and can be exploited for therapeutic interventions.
However, a known snag in biomedical engineering is controlling the delivery of good bacteria to a target site in the human body. Some approaches might include encapsulating the bacteria in synthetic material, like hydrogels, that dissolve over time. But this type of delivery is short-lived.
In a new study, Texas A&M researchers have designed materials that deliver good bacteria over longer durations.
They discovered as probiotic bacteria grow and multiply in the hydrogel, they exert pressure on the material, eventually causing it to fracture. Thus, manipulating the stiffness of the hydrogel could influence how quickly the bacteria divide, form colonies, and then release themselves from the hydrogel.
These findings are published in the journal .
At appropriate doses, good bacteria offer several benefits to host organisms, including modulating gut microbiome, influencing immune responses and fighting pathogens. The most well-known example of probiotics in human health is dietary supplements that can bolster gut health. But unlike the gut, other areas of the body can be challenging to reach.
"For the gut, you can take a supplement or eat food containing probiotics, but you can imagine that the bladder, a common site for urinary tract infections, can't be directly reached by ingesting probiotics," said Dr. Taylor Ware, associate professor in the Department of Biomedical Engineering and Materials Science & Engineering.
For these hard-to-reach areas of the body, microorganisms can be delivered using hydrogels, materials similar to those used in soft contact lenses. Hydrogels serve as ideal platforms to encapsulate bacteria and act like on-site probiotic factories, enabling bacteria to grow in the hydrogel at the target site. These bacteria then begin to escape the hydrogel steadily but then quickly run out.
"I think the big challenge is sustained release, that is, not just getting the bacteria to the area of interest but keeping the device there and delivering the bacteria at a controlled rate for a long period," said Ware.
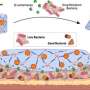
To investigate the mechanisms of microbial release, the researchers embedded different good microorganisms, such as Escherichia coli and yeast, in soft hydrogels. They genetically altered the bacteria to glow when viewed under a microscope, allowing researchers to track whether the bacteria remained in the hydrogel as they colonized the material or were released.
Researchers then placed the bacteria-laden hydrogel in a cultivating medium to encourage microbial growth. They found that after a specific duration, the growing colonies of bacteria caused small cracks in the hydrogel, eventually escaping into the surrounding environment.
"As the bacteria grow and proliferate, they push out into the surrounding environments, and when they do, eventually, they cause that material to break," said Ware. "The entire material doesn't split into pieces; instead, tiny fractures occur, providing a path for cells to travel through to the surface and release themselves."
The researchers found that tweaking the mechanical properties of the hydrogel, for example, by making it stiffer, they could control bacterial growth and release.
"There's very limited work that's been done on delivering microorganisms using synthetic materials," said Ware. "Our research is an important step in developing medical devices that can release bacteria over a sustained period to alter the course of infections without the use of antibiotics."
More information: Manivannan Sivaperuman Kalairaj et al, Controlled Release of Microorganisms from Engineered Living Materials, ACS Applied Materials & Interfaces (2025).
Journal information: ACS Applied Materials and Interfaces
Provided by Texas A&M University