Direct plasma membrane-to-ER lipid transfer outpaces vesicular trafficking, study reveals
Justin Jackson
сontributing writer

Sadie Harley
scientific editor

Robert Egan
associate editor

Max Planck Institute of Molecular Cell Biology and Genetics led a study showing that directional, non-vesicular lipid transport drives fast, species-selective lipid sorting, outpacing slower, less specific vesicular trafficking, and yielding a quantitative map of retrograde lipid transport in cells.
Thousands of lipid species occupy distinct organelle membranes, with task differences that determine cellular function. Gaps in live-cell imaging capabilities have limited clarity on how individual lipids move between organelles to maintain those tasks.
Biosynthesis of lipids begins in the endoplasmic reticulum (ER), followed by distribution toward the plasma membrane and subsequent recycling back into the ER or catabolism in lysosomes, peroxisomes, and mitochondria.
Previous studies have tracked some anterograde flow using metabolic labeling and fractionation, while retrograde pathways for individual species remain less defined, with sphingomyelin as a noted exception.
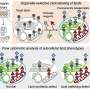
In the study, "Quantitative imaging of lipid transport in mammalian cells," in Nature, researchers measured retrograde transport and metabolism of individual lipid species using time-resolved fluorescence imaging of bifunctional lipid probes combined with ultra-high-resolution mass spectrometry and mathematical modeling.
U2OS cells served as the main system for lipid loading and imaging, with HCT116 cells and genetic knockdowns used for mechanistic tests. Researchers slipped bifunctional lipids into the plasma membrane using a cyclodextrin carrier, then locked them in place with ultraviolet light and tagged them chemically for visualization.
The probes reported both location and metabolic fate, spanning phosphatidylcholine, phosphatidic acid, phosphatidylethanolamine, and sphingomyelin, with variants differing in chain saturation and position.

Imaging assigned lipid signals to specific organelles, while mass spectrometry tracked their chemical conversions. Mathematical models compared direct plasma membrane to ER transfer with vesicular routes through endosomes and the Golgi. Drug treatments and gene knockouts probed how flippases and transport proteins contribute to these flows.
Polyunsaturated phosphatidylcholine, phosphatidic acid, and phosphatidylethanolamine appeared in the ER almost immediately after loading, while saturated phosphatidylcholine and sphingomyelin lingered at the plasma membrane and in endosomes. Direct plasma membrane to ER transport outpaced vesicular movement through endosomes by as much as 11-fold.
Phosphatidylethanolamine traveled fastest, followed by polyunsaturated phosphatidylcholine and sphingomyelin, with saturated phosphatidylcholine moving slowest. Within the phosphatidylcholine group, unsaturated species moved up to seven times faster than saturated ones, and chains placed at the sn-2 position traveled up to twice as fast as those at sn-1.
Blocking vesicular trafficking did not prevent lipids from reaching the ER at early timepoints, though sphingomyelin later built up in endosomes. Removing CPTP cut sphingomyelin delivery to the ER by nearly half, with smaller effects on other classes. Knocking down the flippase subunit TMEM30A slowed phosphatidylethanolamine transport threefold and reduced the balance of forward to backward flow.
Transport proved much faster than metabolism, with rates exceeding chemical conversions by factors of ten to sixty. Even so, transport and metabolism were tightly linked, especially within phosphatidylcholine.
Over longer periods, sn-1-modified phosphatidylcholine generated more lipid droplets and cholesterol esters than sn-2 forms, while triacylglycerol production remained similar across variants.
Findings indicate that non-vesicular transport dominates organelle lipid distribution, with coupling between ATP-dependent lipid flipping and selective transport offering a mechanism for directional movement.
Species-specific phospholipid metabolism regulates neutral-lipid accumulation. The implications span mechanism, modeling, target discovery, and lipid-storage biology, with a reliable atlas to power follow-on work.
Written for you by our author , edited by , and fact-checked and reviewed by —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Juan M. Iglesias-Artola et al, Quantitative imaging of lipid transport in mammalian cells, Nature (2025).
Journal information: Nature
© 2025 Science X Network