Triggering RNA activation on demand: Strategy expands options for therapeutics and gene editing

Sadie Harley
scientific editor

Robert Egan
associate editor

National University of Singapore (NUS) researchers have devised a method to safely and temporarily "switch off" and then "turn on" ribonucleic acid (RNA) inside cells. This is achieved using structurally optimized disulfide-containing chemical groups that attach to RNA and keep it inactive until conditions inside the cell naturally remove these groups, restoring normal RNA function. This strategy could potentially open new avenues in more precise RNA-based therapeutics and gene editing.
The findings are published in the journal .
RNA has gained prominence as a next-generation therapeutic platform. However, it is still challenging to deliver RNA safely to the right place in the body and activate it only when and where it is needed. Existing RNA delivery methods, like lipid nanoparticles, face limitations including possible side effects, limited efficiency, and lack of precise control.
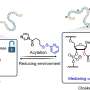
To address these issues, a research team led by Assistant Professor Zhu Ru-Yi from the Department of Chemistry at NUS developed a purely chemical, post-synthetic approach that temporarily "cages" RNA by modifying its 2′-OH sites with carefully tuned disulfide-containing acyl groups.
These modifications can temporarily block the RNA from carrying out its natural biological activity until intracellular glutathione (GSH), a common reducing agent, acts as the "key" by triggering a redox reaction that removes the acyl groups.
By adjusting the chemical structure and properties of these acyl groups, the researchers can achieve fast, efficient, and controllable RNA activation for diverse RNA types, from short synthetic strands to long messenger RNAs (mRNAs).
Assistant Professor Zhu said, "Our approach provides a universal method to modulate RNA activity with spatial and temporal control, without relying on enzymes or light. This is the first example of such responsive mRNA activation that has been shown to work in both test tube and live-cell environments."
Through a series of systematic optimizations, the team established three distinct chemical methods to acylate RNA post-synthetically. These methods enable reversible blocking of the RNA function and can be triggered to release RNA by either natural or externally supplied GSH.
The modified RNA demonstrated excellent stability, selective activation, and successful functional recovery in vitro and in living cells. Notably, when applied to CRISPR-Cas9 gene editing and mRNA translation, the redox-responsive RNAs were able to fully regain their activity upon GSH treatment, highlighting the system's versatility.
Importantly, this strategy does not rely on bulky delivery systems or potentially harmful external triggers, making it appealing to future applications in synthetic biology, RNA therapeutics, and intracellular delivery.
"The simplicity and broad compatibility of our redox-responsive acylation system make it accessible to a wide range of researchers working with RNA," added Assistant Professor Zhu.
Looking ahead, the team is designing new chemical tools and responsive RNA modifications to further refine the control over RNA activity in living systems. Their goal is to enable more precise, programmable RNA-based therapies for future medical and research applications.
More information: Junsong Guo et al, Accelerating Responsive RNA Release Through Structural Optimization of Disulfide‐Containing Acyl Groups, Angewandte Chemie International Edition (2025).
Journal information: Angewandte Chemie International Edition
Provided by National University of Singapore