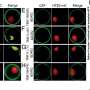
When cells produce aGPCRs—our cellular force sensors—the receptors split into two parts. It has now been shown that they need assistance to complete this cleavage before being transported to the cell surface. Credit: Yin Kwan Chung
Researchers at Leipzig University and Martin Luther University Halle-Wittenberg have investigated a previously unknown process that occurs during protein synthesis in the cell. They examined how so-called adhesion G protein-coupled receptors (aGPCRs) split themselves into two parts.
This self-cleavage takes place in a region of the protein known as the GAIN domain, which is considered crucial for the receptor's ability to detect and transmit signals. The self-cleavage acts as a kind of built-in quality control: only correctly cleaved receptors are allowed to leave the "cell factory" and reach the surface.
The study is published in the journal .
Cells constantly monitor their surroundings and detect chemical and mechanical signals that control vital functions such as movement, growth and communication. aGPCRs—a special family of surface molecules—act as mechanical force sensors and play a key role in processes ranging from muscle growth to the formation of neural networks in the brain.
Malfunctioning aGPCRs can cause serious health conditions such as allergies, schizophrenia, and cancer.
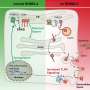
The research teams led by Professor Tobias Langenhan of Leipzig University's Faculty of Medicine and Professor Andrea Sinz from the Institute of Pharmacy at Martin Luther University Halle-Wittenberg discovered that another part of the receptor—the so-called seven-transmembrane (7TM) region—plays a crucial supporting role in the cleavage process.
It not only stabilizes the GAIN domain but also helps position it correctly within the cell's protein-producing machinery. In addition, the teams identified helper molecules in the cell that interact with the receptor during this process. These include enzymes that add sugar groups to the newly formed protein.
"Together, these factors ensure that the receptor's self-cleavage proceeds efficiently. Remarkably, receptors that are unable to cleave themselves can be retained within the cell and fail to reach the surface, where they are needed to receive signals from the external environment," says Professor Tobias Langenhan.
These findings suggest that self-cleavage functions as a built-in quality control mechanism within the cell. This finding opens up new avenues of research into how this checkpoint is implemented and what role it plays in the development of certain medical conditions.
More information: Yin Kwan Chung et al, Self-cleavage of the GAIN domain of adhesion G protein-coupled receptors requires multiple domain-extrinsic factors, Nature Communications (2025).
Journal information: Nature Communications
Provided by Leipzig University