Credit: Nature Sustainability (2025). DOI: 10.1038/s41893-025-01665-y
Scientists have long noted with interest that in one of nature's crucial chemical reactions—the oxygen evolution reaction—it is manganese, rather than more plentiful similar elements such as iron, that acts as the key catalyst.
Now, in research published in , a research group led by Ryuhei Nakamura at the RIKEN Center for Sustainable Resource Science (CSRS) in Japan has discovered that manganese has a unique ability to act even when there are fluctuations in electrical voltage, and that this ability is critical to its success as a catalyst.
This also means that artificially, manganese is a good candidate for applications, such as in wind and solar energy, in which the electrical output fluctuates.
The oxygen evolution reaction, which uses energy to liberate protons and electrons from compounds such as water—freeing oxygen in the process—is critical to life on Earth as it functions at the core of photosynthesis. It is the free oxygen produced in this reaction that allows us and other life forms to get energy by breathing.
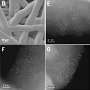
Manganese acts as the main catalyst in this reaction, in the form of an oxide-like manganese cluster, which can exist in a variety of oxidation states. When solar or electrical energy fluctuates, manganese's oxidation state changes, allowing it to repeatedly drive the reaction.
The cyclic nature of this process is a key feature for sustainable reactions, as it allows catalysts to be used over and over. For an ideal catalyst, this process would be repeated indefinitely.
However, real catalysts deactivate over time, for example, due to the dissolution of metal ions such as manganese. Normally, this is a one-way path and the lost ions do not return to the catalyst.
For the current study however, the researchers incorporated the Guyard reaction—in which a manganese ion with an oxidation state of 7 is converted to one with a state of 3. The researchers found that while the catalyst decomposes as expected when the voltage gets too high, with the incorporation of the Guyard reaction, it is regenerated when the excess voltage is removed.
Specifically, when the researchers repeatedly switched the voltage back and forth between 1.68 volts and 3.00 volts, the catalyst was able to maintain a current of 250 milliamperes per square centimeter at a pH of 2 for more than 2,000 hours, highlighting the importance of pathway design for sustainable energy conversion from intermittent renewable sources.
This regeneration is unique to manganese, as similar chemical elements in the "3d block," such as cobalt, iron, and nickel, cannot regenerate under the same experimental conditions. The authors believe this is a major factor in why manganese came to be the key catalyst in photosynthesis.
In terms of impact, according to Nakamura, "We have shown it is possible to develop materials resistant to fluctuating voltages, with the potential to eventually develop materials that can be used in water electrolyzers—devices that convert water into oxygen and hydrogen—connected directly to renewable energy sources."
This is because renewable energy sources, such as solar and wind power, fluctuate on time scales of seconds to hours.
Moving forward, Nakamura states, "This is important work, but in order to create industrial applications for this, we would need to be able to extend the lifespan by at least an order of magnitude. We are tackling this issue now."
More information: Ailong Li et al, Oxygen evolution electrocatalysis resilient to voltage fluctuations, Nature Sustainability (2025).
Journal information: Nature Sustainability
Provided by RIKEN