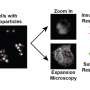
3D slicer MRI image manual segmentation. Rat MRI image from https://openneuro.org/datasets/ds002870/versions/1.0.1 Credit: Jaison Jeevanandam
When I first realized the scale of the challenge posed by neurodegenerative diseases, such as Alzheimer's, Parkinson's disease and amyotrophic lateral sclerosis (ALS), I felt simultaneously humbled and motivated. These disorders are not caused by a single malfunction in the system, but rather by a cascade of failures, which includes protein misfolding, synaptic breakdown, impaired repair mechanisms and poor drug delivery across the blood-brain barrier.
My research at the National Institute of Mental Health (Czechia) along with my collaborators from Spain, the United Kingdom and the United States of America (U.S.) set out to ask a bold question: what if we combined smart nanomedicine with molecular imaging and artificial intelligence (AI/ML) to create a new generation of therapies?
In our recent review article in Molecular Diagnosis & Therapy, we explore the possibility of nanomedicine and AI synergy in neurodegenerative disease treatments.
Why nanomedicine matters
Traditional drug delivery to the brain faces several barriers, such as the physiological barrier of the skull and meninges, the blood–brain barrier (BBB), rapid clearance of small molecules, and off-target toxicity.
Nanomedicines, precisely engineered particles at the nanoscale, offer ways around these obstacles via targeted delivery, controlled release, and improved bioavailability. However, engineering of particles is only half the battle. Without real-time imaging and intelligent feedback, we're shooting in the dark.
Where imaging and AI enter the equation
That's where molecular imaging (MRI, PET, and contrast agents) meets a machine learning approach. We can monitor "where nanomedicines go, how much reaches the target tissue, and what biological effect it has," by incorporating imaging data and training AI/ML models.
In our review, we have provided detailed information on how imaging-based biomarkers and ML algorithms can substantially help in optimization of nanomedicine frameworks, such as selecting particle size, coating, targeting moieties and release kinetics.
My personal journey
In our lab, I recall when one of our nanoparticle formulations looked promising in vitro, but when it reached animal tissues in vivo, the distribution was unexpectedly sparse. We could trace that gap using imaging processes.
Later, we analyzed patterns of nanoparticle uptake across regions with AI and adjusted the particle architecture accordingly. It's this cycle, design → image → AI-driven feedback → redesign, that defines the successful interdisciplinary approach for the patient-centric treatment of neurodegenerative diseases.
Key takeaways
- Multimodal targeting: Nanocarriers must cross the BBB, reach affected neurons and release cargo at the right time, where image processing helps to validate each step.
- Personalized optimization: AI models trained on patient or animal imaging datasets can help predict which formulation works best in the desired biological context.
- Real-world translation: We emphasized the need for clinical-grade imaging data, robust ML pipelines that handle patient heterogeneity, and manufacturing constraints aligned with regulatory frameworks.
- Challenges ahead: We are frank about the challenges in the review, which includes particle safety, long-term accumulation, immune responses, scalable manufacturing, the need for shared ML imaging datasets and open standards.
What this integration of AI and nanomedicine means for patients and the field
It might sound futuristic, but I believe we're at the cusp of a new era, where nanomedicine combined with molecular imaging and AI could shift from "manage disease" to "modify disease."
For patients living with cognitive decline or motor neuron loss, that shift is huge. As I reflected in the review, what we really need is to empower the brain's resilience, not just treat symptoms.
The next steps in our work
My team is working on a next-generation nanoformulation named molecular nanorobots as nasal spray and ML algorithms to predict the specific dosage required for neurodegenerative disease treatment.
Further, we're also building an AI model that uses imaging patterns from Alzheimer's and Parkinson's cohorts to refine delivery parameters. In parallel, we are collaborating with clinicians to define imaging endpoints that matter for regulatory approval.
I'm writing this dialogue because I want you, the reader, whether a graduate student, clinician, or nanotechnologist, to feel part of this journey.
This is not just a paper; it's a call to interdisciplinary action. If you work in molecular image processing, think of how your data can inform nanoparticle design. If you work in ML, think of how your models can optimize delivery rather than just classify images.
If you're in the field of nanomedicine, it is time to partner with imaging experts and AI specialists. In our lab, we are currently synthesizing novel nanoparticles and developing AI models capable of predicting optimal nanomedicine dosages from MRI images of mouse brains.
We are eager to expand this work through collaborations with clinicians, imaging specialists, and experts in nanoparticle characterization techniques. Together, we can accelerate the translation of nanomedicine and AI into meaningful neurological therapies.
A final thought
Sometimes I imagine a future where a patient diagnosed early with neurodegeneration receives a nanomedicine infusion, we take an MRI, feed the image into an AI model, adjust the next dose, and fine-tune therapy like we adjust a musical instrument.
That may sound idealistic, but our review shows that the building blocks are already in place. We just need to build the bridges between materials science, imaging, AI, and clinical practice to make it a reality.
This story is part of , where researchers can report findings from their published research articles. for information about Science X Dialog and how to participate.
More information: Jaison Jeevanandam et al, Smart Nanomedicines for Neurodegenerative Diseases: Empowering New Therapies with Molecular Imaging and Artificial Intelligence, Molecular Diagnosis & Therapy (2025).
I am Jaison Jeevanandam, Ph.D., C.Sci., MRSB, a MERIT fellow in the Division of experimental neurobiology, Preclinical research program, National Institute of Mental Health, Czechia. My research focuses on developing smart nanocarriers for neurological disorders, integrating molecular imaging, machine learning, and translational nanomedicine. I aim to steer nanomedicine from bench to bedside with interdisciplinary collaborations spanning U.S., Europe, Asia and Australia.