How protein coatings influence nanoparticles' ability to avoid immune clearance and reach their destinations

Lisa Lock
scientific editor

Robert Egan
associate editor

Nanomedicine uses ultra-small particles to deliver drugs directly to the tissues and cells that need them, improving treatment effectiveness while reducing side effects. This approach underpins some clinically approved RNA-based technologies and chemotherapy drugs.
But designing effective nanomedicines is no easy task. The body's immune system can mistake nanoparticles for harmful invaders and try to clear them, limiting their effectiveness and sometimes causing adverse effects. A key player in this process is the protein corona, a layer of proteins that forms around nanoparticles when they enter the bloodstream that can influence how the immune system reacts to them.
Findings from researchers at the University of Delaware College of Engineering, Sept. 29 in the Proceedings of the National Academy of Sciences, offer fresh insights into how the protein corona affects immune system recognition of nanoparticles and the particles' ability to reach their destination.
"Understanding the influence of the protein corona on a nanoparticle's fate will help us design nanomedicines that more reliably evade immune clearance and deliver therapies precisely," said senior author Emily Day, professor in the Department of Biomedical Engineering.
Targeting blood stem cells
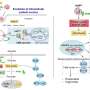
For this study, a team from Day's laboratory focused on nanoparticles targeting hematopoietic stem cells, which give rise to all types of blood cells. Nanomedicines that deliver drugs or gene therapies directly to these cells could serve a range of purposes, from helping prepare the body for a bone marrow transplant to correcting the genetic variation that causes sickle cell disease.
"The rarity of hematopoietic stem cells—they comprise just 0.01% of bone marrow cells—makes them a challenging target," said first author Eric Sterin, who received his doctorate in biomedical engineering from UD earlier this year. "One approach we've been using to improve targeted delivery is wrapping the nanoparticles with a membrane derived from bone marrow cells known as megakaryocytes, which coats them with proteins that help guide them to the marrow."
The team tested how these membrane-wrapped nanoparticles behave in laboratory and animal experiments. To form protein coronas, they incubated the particles in blood serum from mice, cows or humans. Compared to unwrapped nanoparticles, membrane-wrapped particles bound less protein overall, and in human serum the classes of proteins that bound to them were especially distinct.
Further experiments suggested that the sparser protein coronas on the membrane-wrapped particles may influence how they interact with cells. In lab studies, these particles entered their target cells more easily and were less likely to be taken up by immune cells than the unwrapped particles.
Exploring the protein corona
The researchers next sought to figure out which proteins in the corona have the biggest impact on a nanoparticle's fate. Understanding this could show them which parts of the corona to adjust to make nanomedicines more precise.
Proteomics analyses revealed that apolipoprotein B was the most abundant protein in the corona. This was somewhat surprising, as other studies with similar nanoparticles had found apolipoprotein E to be more common. Both proteins help carry molecules around the body, but they can also act as "flags" that make it easier for clearance cells to spot and remove the particles.
To further explore how individual proteins in the corona affect nanoparticle destiny, the researchers used different mouse models, each engineered to lack one specific type of protein. Examining where the particles accumulated in mice after injection into the bloodstream revealed an intriguing and delicate balance. Some proteins in the corona, such as complement component 3 and immunoglobulin G, were found to help the immune system clear particles to organs like the liver. But those same proteins can also help the particles reach the targeted hematopoietic stem cells in the bone marrow.
"Finding ways to control the levels of these proteins could help shift the balance toward more precise delivery to blood stem cells in the bone marrow and less off-target delivery to other organs," said Day.
Currently, the researchers are exploring how to adjust the composition of the protein corona by altering components of the membrane wrapping. A future direction is to extend their experiments to humanized mice, which have a human-like immune system, to better understand how these nanoparticles might behave in people.
More information: Eric H. Sterin et al, Influence of the protein corona on hematopoietic stem and progenitor cell uptake and macrophage clearance of membrane-wrapped nanoparticles, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Delaware