Researchers reveal competitive mechanism of dual-mode nitrogen fixation in metal carbide clusters

Lisa Lock
scientific editor

Robert Egan
associate editor

Contemporary industrial nitrogen fixation largely relies on the energy-intensive Haber-Bosch process, which operates under extremely high temperatures and pressures.
Metal carbides, as a promising class of catalytic materials, have recently attracted attention in heterogeneous catalysis research. Understanding their nitrogen activation mechanisms at the molecular and electronic levels is crucial for developing next-generation catalysts and advanced single-atom materials.
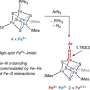
In a study in Chemical Science, a research team led by Prof. Jiang Ling and Prof. Xie Hua from the Dalian Institute of Chemical Â鶹ÒùÔºics (DICP) of the Chinese Academy of Sciences revealed the competitive mechanism of dual-mode nitrogen fixation in a negatively charged metal tricarbon cluster, MC3– (M = Os, Ir, Pt).
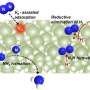
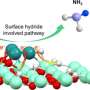
Combining photoelectron spectroscopy with quantum chemical calculations, researchers investigated the reactivity of MC3– clusters in activating nitrogen molecules. They demonstrated that these clusters exhibited two competing nitrogen activation pathways: cleavage of the N≡N triple bond with the formation of a stable C–N bond, and chemisorption of nitrogen onto the metal center.
Researchers found that among the studied clusters, OsC3− primarily facilitated N≡N bond cleavage, IrC3− displayed the coexistence of dual nitrogen activation mechanisms, and PtC3− favored nitrogen fixation mainly through chemisorption.
Furthermore, theoretical analysis revealed that the activation of nitrogen by MC3– decreased as the 5d orbital energy of the metal atoms lowered, while the predominance of chemisorption correspondingly increased.
"Our study provides molecular-level insights into dinitrogen activation by mononuclear metal carbide clusters, and establishes a new paradigm for developing efficient catalysts for dinitrogen fixation," said Prof. Xie.
More information: Shihu Du et al, Observation of competing nitrogen activation in metal tricarbon anions MC3− (M = Os, Ir, Pt), Chemical Science (2025).
Journal information: Chemical Science
Provided by Chinese Academy of Sciences