Custom-designed polymers open new path to electrochemical separations for sustainable drug manufacturing

Gaby Clark
scientific editor

Robert Egan
associate editor

Enantiomers, or molecule pairs that are mirror images of each other, make up more than half of FDA-approved drugs in use today, including those used in treatments for cancer, neurologic diseases and arthritis. Separating enantiomers is critical for drug manufacturing because the effect of each molecule in the pair can be very different—for example, one enantiomer might cure a headache while its mirror-image could cause a headache.
Faster and more accurate enantiomer separations would help with the overall drug discovery and screening process, but by their very nature, enantiomers—which have identical compositions and only differ by not being superimposable (think left hand and right hand)—are notoriously difficult to separate.
An effort by a group of researchers at the University of Illinois Urbana-Champaign to find an efficient, sustainable way to perform these critical enantiomer separations is the focus of a new study in the Journal of the American Chemical Society.
"This is one of the hardest separations in the world," said Xiao Su, a chemical and biomolecular engineering professor at Illinois who led the project. "We're talking about separating almost identical chemical molecules from one another."
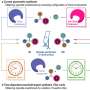
"The way to separate enantiomers is to introduce a chiral environment that can 'recognize' the difference between the two mirror images of the enantiomers," explained Jemin Jeon, co-first author on the paper and former Ph.D. student under Su.
"Conventional processes for separating these enantiomers often leads to a large amount of chemical waste. We wanted to achieve a more sustainable but still effective enantioselective separation by developing a chiral interface that can selectively capture one enantiomer over the other and be turned on and off by electricity."
Although electrochemical separations have been used successfully for many ion recovery processes, performing enantiomer separations has so far been unachievable because of the lack of proper redox-responsive polymer adsorbents that attract and bind enantiomers. Solving this problem was a key goal of the researchers.
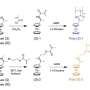
The team focused on ferrocene as the molecular building blocks of their materials due to its ability to carry out redox reactions—that is, to accept and donate electrons. Uniquely, they introduced methyl and selenium phenyl groups into the ferrocene's molecular structure to create polymers with planar chirality, where the chiral elements are arranged in a 2D plane. These chiral ferrocene units form the basis for achieving selectivity for enantiomers, with the added benefit of being able to turn on/off interactions solely through electrochemical control.

While Su and Jeon have previously shown how chiral ferrocenes can be used for sensing and molecular recognition, these prior instances were restricted to point-chirality, where the chiral center is around a central atom. Here, their work showed that new planar chiral ferrocenes resulted in significantly better enantioselectivity than point chiral ferrocenes, thus enabling planar chiral polymer platforms to perform enantioselective separations as electrosorbents.
In fact, when they carried out the electrochemical separation process using the new synthesized polymers, they found that a targeted enantiomer could successfully be separated from a racemic, or 50–50, mixture of enantiomers. The work also showed how engineering design can theoretically bring the target enantiomers to 99% purity.
"The uniqueness of these polymers is that they are not only chiral, but they're chiral and electrochemically responsive," Su said. "It's a completely new application for electrochemical separations."
Su emphasized that the ability to do the separations electrochemically not only brings greater efficiency to the drug screening and manufacturing process, but also has significant environmental benefits.
"Pharmaceutical separations can often be very costly and chemically wasteful," he said. "The separation of these enantiomers requires large systems that use a lot of solvent and which generate a lot of chemical waste. By doing this process electrochemically, you can reduce the waste and the chemicals that are being used."
While this study focused on separations of enantiomeric pairs of amino acids, Jeon said there are countless potential applications of their work.
"This is just a beginning for the design of redox-active chiral interface and electrochemical systems," Jeon said. "We believe there are unlimited opportunities in pursuing these concepts for the enantioselective separations of a wider group of molecules, including valuable pharmaceuticals."
Yuri Kappenberg, doctoral student in chemical and biomolecular engineering at Illinois, is the other co-first author on the study. Collaborators include U. of I. chemical and biomolecular engineering professor Alex Mironenko and visiting chemistry professor Fabio Zazyki Galetto from Universidade Federal de Santa Catarina in Brazil.
More information: Jemin Jeon et al, Planar Chiral Metallopolymers for Electrochemically Mediated Enantioselective Separations, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by University of Illinois at Urbana-Champaign