Single experiment can measure enzymatic kinetics for over 200,000 possible substrates

Sadie Harley
scientific editor

Robert Egan
associate editor

A pharmaceutical scientist at the National University of Singapore (NUS) has developed a method that can measure the kinetic efficiency of an enzyme against more than 200,000 potential peptide substrates in a single experiment.
Characterizing the interactions between enzymes and their substrates is a fundamental task in biochemistry, essential for engineering new biocatalysts, understanding disease mechanisms, and designing therapeutics. While existing techniques can study many enzymatic reactions in parallel, scaling such methods to comprehensively analyze an enzyme's preferences across a vast space of possible substrates remains a practical challenge.
Assistant Professor Alexander Vinogradov from the Department of Pharmacy and Pharmaceutical Sciences at NUS has developed a strategy called DOMEK (mRNA-display-based one-shot measurement of enzymatic kinetics) that addresses this need.
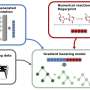
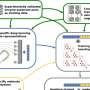
The method combines a technique called mRNA display, which enables rapid preparation of thousands of enzymatic substrates, with next-generation sequencing to calculate a key kinetic parameter, known as the specificity constant (kcat/KM), for each individual substrate in a single experiment.
DOMEK is an operationally simple and generalizable technique that relies on standard molecular biology equipment and requires no engineering expertise. This research work was carried out in collaboration with Professor Hiroaki Suga from the University of Tokyo, Japan.
Beyond measuring kinetics, the large dataset produced by DOMEK enabled the team to leverage statistical modeling to understand how an enzyme recognizes its substrates. This helps in unraveling the source of the extraordinary catalytic efficiency of enzymes and has immediate applications in engineering new biocatalysts and therapeutics.
The study, published in the journal , demonstrates the utility of this approach by conducting a single-shot profiling of a bacterial reductase enzyme, which can be employed to engineer potential therapeutic agents.
The authors reliably monitored enzymatic kinetics for 285,000 distinct peptide substrates and validated the results with traditional methods.
Assistant Professor Vinogradov said, "Our approach provides a way to gather quantitative kinetic data on a scale that was difficult to achieve previously. We are now working to expand and generalize the technique, as well as scale it further to measure millions of reactions in parallel."
Looking ahead, the team aims to adapt the DOMEK framework for use with other classes of enzymes involved in post-translational modifications of peptides and proteins.
More information: Alexander A. Vinogradov et al, Measuring kcat/KM values for over 200,000 enzymatic substrates with mRNA display, Chem (2025).
Journal information: Chem
Provided by National University of Singapore